The Fascinating Reaction of Baking Soda and Sulphuric Acid
Have you ever wondered what happens when you mix baking soda, a common household item, with sulphuric acid, a powerful industrial chemical? It's a reaction that's more than just a simple fizz. This seemingly ordinary combination leads to a fascinating chemical process with practical applications and important safety considerations.
Baking soda, also known as sodium bicarbonate, is a staple in many kitchens. It's used for baking, cleaning, and even deodorizing. Sulphuric acid, on the other hand, is a strong acid used in various industrial processes, including fertilizer production and metal processing. When these two substances meet, a chemical reaction occurs, producing carbon dioxide gas, water, and sodium sulfate.
The reaction between sodium bicarbonate and sulphuric acid has been known for centuries. Early chemists observed the effervescence and studied the resulting products. The reaction's ability to produce carbon dioxide became a key element in early fire extinguishers and leavening agents for baking.
Understanding the chemical equation for this reaction is essential. The reaction can be represented as: 2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2. This equation shows that two molecules of sodium bicarbonate react with one molecule of sulphuric acid to produce one molecule of sodium sulfate, two molecules of water, and two molecules of carbon dioxide gas. This carbon dioxide gas is what causes the visible bubbling and fizzing.
The importance of understanding this reaction goes beyond mere scientific curiosity. It has practical implications in various fields, from understanding the chemistry of baking to industrial applications. The production of carbon dioxide can be harnessed for specific purposes, and the resulting sodium sulfate has its own uses in industries like glassmaking and detergent production.
One practical application of this reaction is in baking. The carbon dioxide released by the reaction between baking soda and an acidic ingredient (like vinegar or lemon juice) helps dough rise. In the case of using sulphuric acid, while not suitable for culinary use, the principle remains the same – the released carbon dioxide creates bubbles that leaven the mixture.
Safety is paramount when dealing with sulphuric acid. It is a corrosive substance and can cause severe burns. Always wear appropriate personal protective equipment, like gloves and goggles, when handling sulphuric acid. The reaction with baking soda also produces heat, so it’s crucial to perform it in a well-ventilated area.
Another important aspect is proper disposal. The resulting sodium sulfate solution should be neutralized before disposal according to local regulations.
Advantages and Disadvantages of Using Sulphuric Acid with Baking Soda
| Advantages | Disadvantages |
|---|---|
| Rapid CO2 production | Highly corrosive and dangerous |
| Complete reaction | Requires specialized handling and disposal |
It is important to remember that reacting baking soda with sulphuric acid is not a common household practice and should only be conducted in controlled laboratory settings with proper safety precautions.
Frequently Asked Questions:
1. What happens when baking soda reacts with sulfuric acid? - Carbon dioxide gas, water, and sodium sulfate are produced.
2. Why is this reaction important? - It demonstrates a fundamental chemical principle and has practical applications.
3. Can I use this reaction in baking? - Using sulfuric acid in baking is dangerous and not recommended. Baking soda is used with safer acidic ingredients.
4. What are the safety precautions? - Always wear protective equipment and work in a well-ventilated area.
5. How do I dispose of the products? - Neutralize the solution and dispose of it according to local regulations.
6. What is the chemical equation for this reaction? - 2NaHCO3 + H2SO4 → Na2SO4 + 2H2O + 2CO2
7. What are the industrial uses of this reaction? - The principles are applied in various chemical processes, though using sulphuric acid with baking soda specifically is less common.
8. Is this reaction exothermic? - Yes, the reaction produces heat.
In conclusion, the reaction between baking soda and sulphuric acid is a fascinating chemical process that exemplifies a fundamental principle in chemistry. While the reaction has historical significance and practical implications, it's essential to prioritize safety. Understanding the chemical equation, the products formed, and the necessary safety precautions allows us to appreciate the power and potential of this seemingly simple combination. Always exercise caution when working with chemicals and follow appropriate safety guidelines. Further research into the specific applications and safety measures regarding this reaction is encouraged for those interested in exploring its full potential.

Citric Acid Equation With Water | Taqueria Autentica

Baking Soda Acid Or Base | Taqueria Autentica

Write chemical formula of the following important compounds Common | Taqueria Autentica

Great Ammonia Plus Sulphuric Acid Balancing Equations Practice Problems | Taqueria Autentica

Solved What Would Be The Proper Mechanism Of The Reaction | Taqueria Autentica

1 g of solid sodium chloride is taken in a clean and dry test tube and | Taqueria Autentica

Soda Acid Fire Extinguisher Diagram | Taqueria Autentica

Reactions of Acids and Bases | Taqueria Autentica
Solved PART B BAKING SODA begintabular | Taqueria Autentica

Baking Soda Science Printables Chemical Reaction | Taqueria Autentica
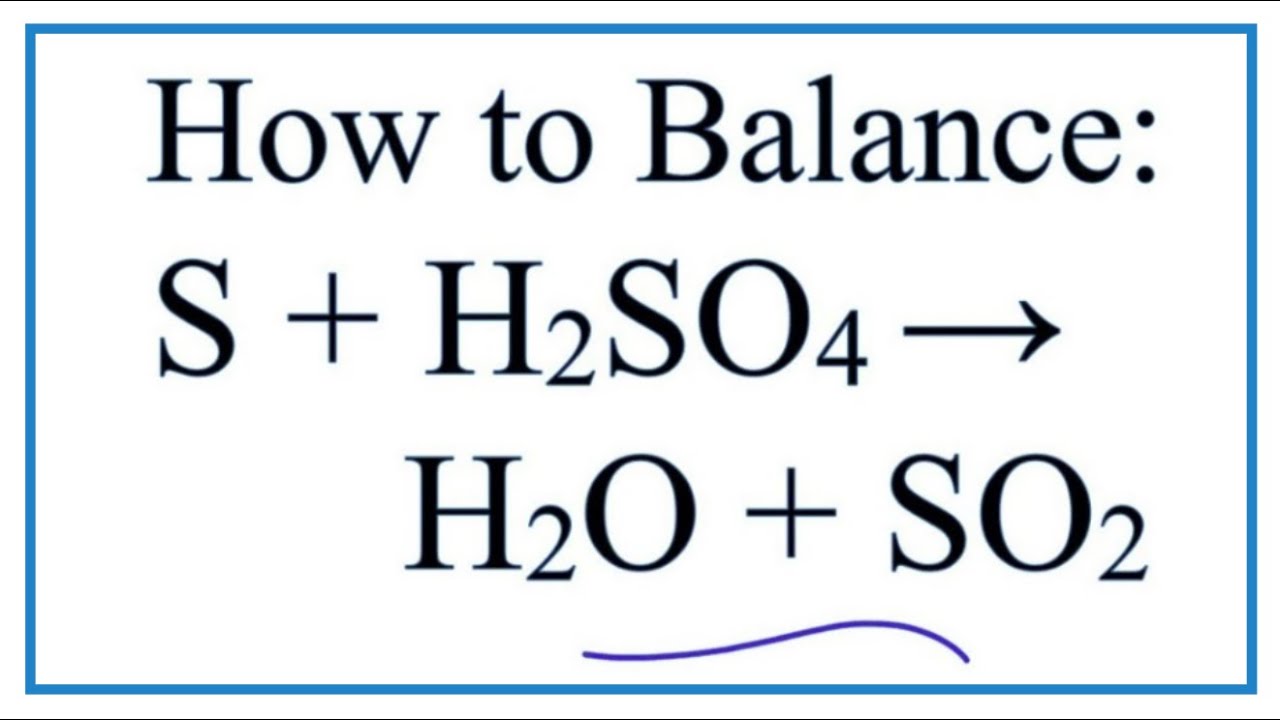
Ace Ammonia And Sulphuric Acid Balanced Equation Definition Of Double | Taqueria Autentica

baking soda + sulphuric acid | Taqueria Autentica

Reaction of citric acid with baking soda | Taqueria Autentica

baking soda + sulphuric acid | Taqueria Autentica

Sand lt baking soda lt sulphuric acid lt glucose lt sugar | Taqueria Autentica